integrative medicine
Pharmacokinectics and Pharmacodynamics
A Pharmacokinetic Primer: How Your Body Processes the Drugs You Take
Drug interactions are an important factor to understand when and how it can happen. The knowledge is crucial to minimize potential drug interactions with some basic knowledge of the human body’s metabolic process upon ingestion of drug, food, beverage, etc.
By understanding pharmacokinectics you will minimize unnecessary agony and worry about “potential” drug interactions. First and foremost, when a research is conducted in vivo or in-vitro, it does not mean the same or similar interaction will “always” take place in it your body. There is a possibility of a same or similar probability of an interaction occurring in your body. The results are then conducted in humans.
In vivo means cell culture or tissue, whereby drugs are tested on a cell culture or tissue from the human body. A single isolated cell culture or lab grown cell culture is not always a 100% representation of what will “always” happen in the human body. By the same principle, in-vitro is study done in a test tube. Again what happen in a test-tube cannot be always equated to happen in a human body.
At any given moment there are hundreds of various chemical activities going on in the human body. It depends on many factors, what were the substances, was it taken as a tea, powder, pill, standardized, taken with food, fat solubility, taken on empty stomach, or taken with other pills, or taken close to your other medications or six hours after medication.
Pharmacologymeans the study of pharmaceuticals, or drugs, for medicinal use in people.
Pharmacokinetics (PK) involves the relationship between the dose of the drug and the concentration (amount) of the drug in the body. PK observes how drug move around the body. Four key steps involved are: Absorption, Distribution, Metabolism and Elimination
Pharmacodynamic is the relation between two or more drugs and how it affects a common physiological system, e.g. the use of Norvir as boosters to increase protease drugs levels.
Half–life of drug is the length of time it takes a drug blood concentration to decrease by one-half. Some HIV drugs like sustiva and tenofovir have long half-life. Sustiva has a half-life of three to five days and tenofovir has a half–life of seven to 10 days.
PK studies are important because; to be aware of how HIV drugs and other conditions affect each other and to know how drug concentrations vary under specific circumstances. Finally, to determine the concentrations of HIV drugs or other medications in all areas of the body to ensure effectiveness and therapeutic value.
The four factors that affect PK levels: Absorption, Distribution, Metabolism and Elimination.
Absorption
In order for any drug to work, it needs to find its way into the body through a process known as absorption. This is a 2 step process:
Distribution
Goal of distribution is to get the HIV drug to where it’s needed – inside the CD4 cells. The distribution also involves its intracellular concentration inside cells. The barriers that stand in the way are:
Metabolism
Metabolism is the chemical process by which the body breaks down nutrients and medications. Metabolism takes place in the liver, but can also occur during the absorption stage in the gut. The PK study purpose is to determine how a drug is metabolized and how much active drug remains in the body after it has been prepared for elimination.
Elimination
For some drugs, metabolism in the gut or liver takes care of this, either by making the drug water soluble or fat soluble. However some drugs, including NRTIs, are eliminated by the kidneys.
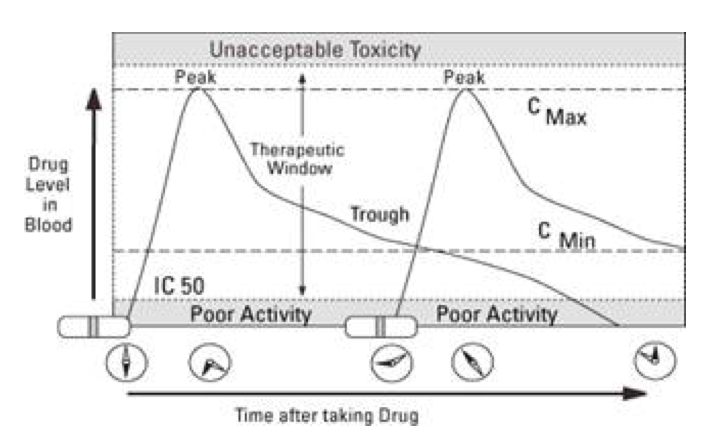
What it means? peak, trough, CMax, CMin
When you take a dose of a medication, the drug level in the blood goes up quickly. In a little while, it reaches its peak. This is called Cmax — the maximum concentration. As the drug is removed (or metabolized) from your body by the liver or kidneys, blood levels of the drug drop. Just before the next dose enters your bloodstream, blood levels of the drug are at their lowest. This is called the “trough,” or Cmin — the minimum concentration.
IC 50
Making Practical Sense of the Information
PK studies are done to ensure two main factors: That enough drug is administered to effectively treat the disease and the drug dose is low enough to avoid or reduce the risk of side effects.
Definitions of terms
- IC50 – The inhibitory concentration (50)
- MEC – Drug’s minimum effective concentration
- Bioavailability – refers to the amount of drug that reaches the blood after it has been administered and initially processed in the gut and the liver
- Cmax – A drug’s maximum concentration in the blood after absorption
- MTC – minimum toxic concentration, the concentration at which unnecessary side effects start to occur
- Tmax – The time it takes for an administered drug to achieve its maximum concentration
- Cmin – The lowest concentration in the bloodstream, before a second dose is taken and increases the concentration.
- AUC – area under the curve, is the total amount of drug maintained in the blood
Further reading
http://hivclinic.ca/drug-information/drug-interaction-tables/
http://kildare.ie/outreach/drugs.htm
http://harmreduction.org/about-us/
http://dean.st/chemsex-support/
For further information and to plan a training session,
please complete GHIS consultation and training request form.

